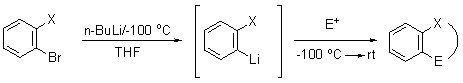
Research Interests:
1. Preparation of Novel Ring Systems Utilizing Parham Cycliacylation/Cyclialkylation Chemistry/Halogen-Lithium Exchange Reactions
During the late 1970’s, Parham and his group found that halogen-metal exchange reactions could be conducted on arene bromides (or iodides) with alkyllithium reagents at low temperatures (ca. -100 o C) functionalized with a variety of electrophilic groups [-CN, -COOH, -COOR, CONR2, -(CH2)n X; attached either directly to the aromatic ring or on a carbon tether adjacent to the exchangeable ring halogen] known to react with alkyllithium reagents at “normal” temperatures1. The remarkable chemoselectivity observed for this reaction has made it possible to pursue the synthesis of compounds which would be exceedingly difficult to prepare by any other means.
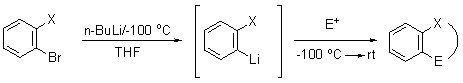
While this powerful technique has been utilized to generate polycyclic ring systems of medicinal interest 2-3, this chemistry, known as the Parham cyclialkylation/cycliacylation reaction, continues to be studied due to its wide applicability and untapped potential for the synthesis of a wide variety of molecular systems3. Many of the ongoing efforts in my laboratory focus on synthetic methods development exploiting the selectivity of Parham chemistry to enable the preparation of novel polycyclic ring systems and highly substituted heterocyclic ring systems4,5.
References
1. Parham, W.E.; Bradsher, C.K. Acc. Chem. Res. 1982, 15, 300-305.
2. Parham, W.E.; Bradsher, C.K.; Hunt, D.A. J. Org. Chem. 1978, 43, 1606-1607.
3. For examples, see (a) Hodgetts, K.J. Tetrahedron 2005, 61, 6860-6870. (b) El Sheikh, S.; Schmalz, H-G.; Curr. Opin. Drug Disc. Dev. 2004, 7, 882-895. (c) Sotomayor, N.; Lete, E. Curr. Org. Chem. 2003, 7, 275-300. (d) Ruiz, J.; Sotomayor, N.; E. Lete, Org. Lett. 2003, 5, 1115-1117. (e) Chen, S.; Plotkin, M.; Spoors, G.P.; Tetrahedron Lett. 2000, 41, 2269-2273. (f) Larsen, S.D. Synlett. 1997, 1013-1014.; (g) Monte, A.P.; Maronalewicka, D.; Parker, M.A.; Wainscot, D.B.; Nelson, D.L.; Nichols, D.E. J. Med. Chem. 1996, 39, 2953-2961. (h) Couture, A.; Deniau, E.; Grandclaudon, P. J. Chem. Soc., Chem. Commun. 1994, 1329- 1330.
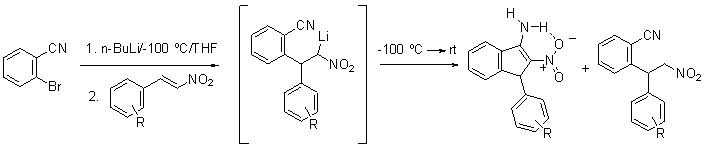
4.
Clarke
A. J.;
Hunt, D.A. “b-Nitrostyrenes
as Electrophiles in Parham Cyclization Chemistry.
Reaction with o-Lithiobenzonitrile“
Tetrahedron Lett. 2009, 50, 2949-2951.
2. Medicinal Chemistry Studies I: Development of Small Molecule Proteasome Inhibitors [with Professor Sudhir Nayak (Biology)].
Cellular proteins are maintained at appropriate levels through a balance between synthesis and degradation. The 26S proteasome helps maintain the appropriate balance through the targeted degradation of proteins and regulates the cell cycle, morphogenesis, differentiation, receptor modulation, DNA repair, and numerous others processes. Since proteasomes are multi-protein complexes which selectively degrade cellular proteins no longer needed, inhibiting them in cancer cells can disrupt protein regulation, thereby leading to apoptosis (programmed cell death). The recent discovery and FDA fast-tracking of PS-341, a synthetic small peptide boronate, and NPI-0052 for development of a treatment regimen for multiple myeloma1 have served to spark interest in the development of easily synthesized small molecules which might inhibit this system as possible chemotherapeutic agents through targeted medicinal chemistry programs.2 The structural diversity between these compounds has led to speculation that there is differential selective inhibition of different proteolytic sites. However, since little is known regarding the actual mode of action at the proteolytic target sites, this is a promising area for mode of action investigations.
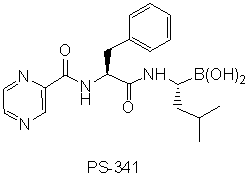
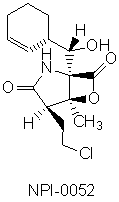
Further, this is a fertile area for the design and development of small molecule inhibitors due to the paucity of compounds developed for chemotherapeutic targeting of the proteasome to date.
References
1. Hideshima, T.; Bradner, J.E.; Wong, J.; Chauhan, D.; Richardson, P.; Shreiber, S.L.; Anderson, K.C. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 8567-8572.
2. Joazeiro, C.A.P.; Anderson, K.C.; Hunter, T. Cancer Res. 2006, 66, 7840-7842.
3. (a) Fiaschi, M.V;
Nayak, S.; Tabakin, E.R.; Hunt, D.A.
“Development of Small Molecule Proteasome Inhibitors,” Biotech 2008
Conference, Philadelphia, PA; November, 2008; Innovation Corridor Session I; (b)
Fiaschi, M.V.;
Stabenow, N.; Tabakin, E.R.; Hunt, D.A.; Nayak, S. “Development of Small
Molecule Proteasome Inhibitors Using
Caenorhabditis elegans,” 17th International C. elegans Meeting
sponsored by the Genetics Society of America, University of California, Los
Angeles, June 25, 2009; No. 1096A.
3. Medicinal Chemistry Studies II: Development of Drugs for a Multi-Paradigm Therapy for Alzheimer ’s Disease [with Professor Richard Egleton (Department of Pharmacology), Dr. James Weinstein (Departmenrt of Neurosciences/Neurosurgery), Dr. Edgar Gonzalez (Pharmacy), Marshall University School of Medicine].
Recent studies have indicated that there are multiple mechanisms involved in the development and progression of Alzheimer’s disease. These mechanisms can be categorized into one of five primary categories: (1) mitochondrial deficiency, leading to increased reactive oxygen species (ROS) formation, (2) an ”immune trigger” of Chlamydophila pneumoniae (90 % of Alzheimer’s patients are brain positive for C. pneumonia), (3) a neurotoxic inflammatory cascade driven by reactive Microglia, (4) generation of reactive oxygen species such as radicals, (5) excessive production of amyloid precursor protein (APP) and its derivative ß-amyloid, leading to synaptic dysfunction and neuronal loss, and(6) ApoE4 allele synthesis leading to toxic fragment formation (60-80% Alzheimer’s patients positive for the ApoE4 allelle). These five pathways combine to form a toxic cycle of neuronal damage and pathology leading to cognitive decline and the clinical presentation of Alzheimer’s disease, and ultimately death. To date most therapies for Alzheimer’s have concentrated on only one of the five causative factors of the disease progression and thus have had only limited success. Our hypothesis is that if a therapy is designed that will treat all five areas of Alzheimer’s disease, this should lead to a much more efficacious treatment strategy that should delay and hopefully potentially reverse the cognitive decline associated with Alzheimer’s. Though there have been considerable advances in drugs that are efficacious in vitro, there remain few viable options for in vivo effective compounds due to the presence of the blood brain barrier (BBB). The BBB plays a significant role in limiting drug entry to the brain and as such is a significant hurdle for Alzheimer’s therapies The focus of this work is to develop drugs that can cross the BBB and treat one or more of the six primary etiologies of Alzheimer’s disease.
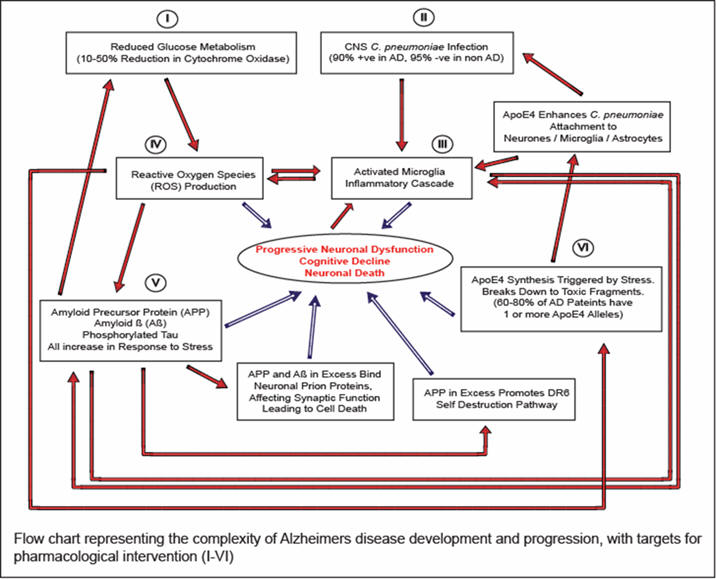
References
1.
Weinstein, J.D.; Gonzalez,
E.R.; Egleton, R.D.; Hunt, D.A. “The 10-patient screening protocol: a paradigm
shift for evaluating pharmacotherapy for Alzheimer’s disease,”
The Consultant Pharmacist
2013,
28, 443-454.
2. Lin, M.T.
and M.F. Beal, Mitochondrial dysfunction
and oxidative stress in neurodegenerative diseases. Nature, 2006.
443(7113): p. 787-95.
3. Swerdlow,
R.H. and S.M. Khan, A "mitochondrial
cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses, 2004.
63(1): p. 8-20.
4. Turrens,
J.F., Mitochondrial formation of reactive
oxygen species. J Physiol, 2003. 552(Pt
2): p. 335-44.
5.
Balin, B.J., et al., Chlamydophila
pneumoniae and the etiology of late-onset Alzheimer's disease. J Alzheimers
Dis, 2008. 13(4): p. 371-80.
6.
Gao, H.M. and J.S. Hong, Why
neurodegenerative diseases are progressive: uncontrolled inflammation drives
disease progression. Trends Immunol, 2008.
29(8): p. 357-65.
7.
Rojo, L.E., et al.,
Neuroinflammation: implications for the pathogenesis and molecular diagnosis of
Alzheimer's disease. Arch Med Res, 2008.
39(1): p. 1-16.
8.
Schwab, C. and P.L. McGeer,
Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders.
J Alzheimers Dis, 2008. 13(4): p.
359-69.
9.
Anandatheerthavarada, H.K., et al.,
Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid
precursor protein impairs mitochondrial function in neuronal cells. J Cell
Biol, 2003. 161(1): p. 41-54.
10.
Devi, L., et al., Accumulation of
amyloid precursor protein in the mitochondrial import channels of human
Alzheimer's disease brain is associated with mitochondrial dysfunction. J
Neurosci, 2006. 26(35): p. 9057-68.
11. Floden, A.M.,
S. Li, and C.K. Combs, Beta-amyloid-stimulated
microglia induce neuron death via synergistic stimulation of tumor necrosis
factor alpha and NMDA receptors. J Neurosci, 2005.
25(10): p. 2566-75.
12.
Lauren, J., et al., Cellular prion
protein mediates impairment of synaptic plasticity by amyloid-beta oligomers.
Nature, 2009. 457(7233): p. 1128-32.
13.
Manczak, M., et al., Mitochondria
are a direct site of A beta accumulation in Alzheimer's disease neurons:
implications for free radical generation and oxidative damage in disease
progression. Hum Mol Genet, 2006. 15(9):
p. 1437-49.
14.
Nelson, T.J. and D.L. Alkon,
Oxidation of cholesterol by amyloid precursor protein and beta-amyloid peptide.
J Biol Chem, 2005. 280(8): p.
7377-87.
15.
Nikolaev, A., et al., APP binds DR6
to trigger axon pruning and neuron death via distinct caspases. Nature,
2009. 457(7232): p. 981-9.
16.
Agosta, F., et al., Apolipoprotein
E epsilon4 is associated with disease-specific effects on brain atrophy in
Alzheimer's disease and frontotemporal dementia. Proc Natl Acad Sci U S A,
2009. 106(6): p. 2018-22.
17.
Bu, G., Apolipoprotein E and its
receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat
Rev Neurosci, 2009. 10(5): p. 333-44.
18.
Pardridge, W.M., Alzheimer's
disease drug development and the problem of the blood-brain barrier.
Alzheimers Dement, 2009. 5(5): p.
427-32.
Michael chemistry of 1,3-diones has been well studied; however, far less is understood about the corresponding 1,2-diones. Previous work by the senior P.I. of this lab has illustrated the synthetic potential of 1,2-diones as precursors for the preparation of heterocycles1:
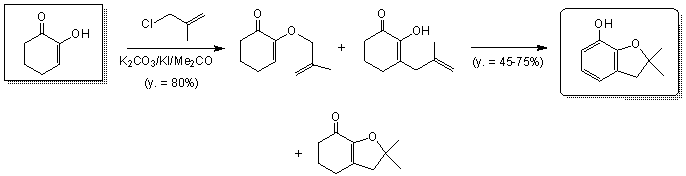
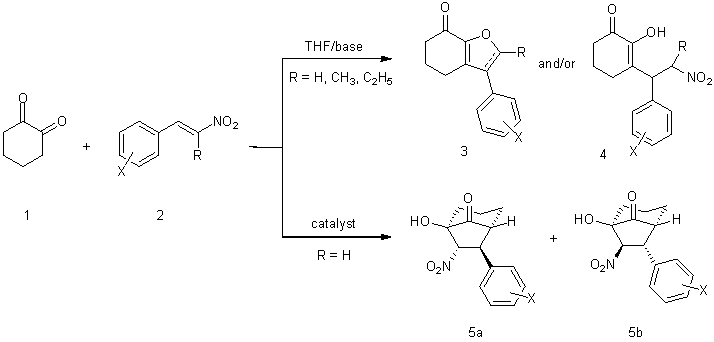
References
1. Hunt,
2. Ding, D.; Zhao, C-G.; Arman, H. Enantioselective synthesis of bicyclo[3,2,1]octan-8-ones using a tandem Michael-Henry reaction. Tetrahedron, 2010. 66, 4423-4427.
Student Research:
Grants
American Chemical Society Organic Chemistry Division – National Travel Grant (2012) - $600
Waters Corporation Academic Grant (2012) - $12,000
George and Rolfa Rogers
Neurodegenerative Diseases Program of Marshall University (2011) - $20,000
NSF MRI CHE-1125993, Co-P.I.: Acquisition of a 400 MHz NMR
Spectrometer for Undergraduate Research and Training (2011) - $261,086.
Marshall University School of Medicine (co-P.I.’s:
James Weinstein, M.D., Professor
of Neurosurgery/ Neuroscience; Dr. Richard D. Egleton, Professor of
Pharmacology; Dr. Edgar Gonzalez, Pharm.D.) –
Development of Drugs and Novel Transport Methods Enabling Penetration of the
Blood-Brain Barrier for the Treatment of Alzheimer’s Disease (2010) - $50,000;
(2011) - $75,000; (2012) - $205,000, (2013) - $2,500.
NSF MRI
CHE-0922931 Co-P.I.: Acquisition of a Single Crystal X-ray Diffractometer for
Undergraduate Research and Training (2009) - $242,200
Phi Kappa Phi Student Research Grant/Award (Sara Davis:
A Convergent Synthesis of (+)-Isopestacin
– 2008) - $500
Merck/AAAS Undergraduate Science Research Co-P.I. (2007-2009): Project:
“Development of Small Molecule Proteasome Inhibitors” -
Sudhir Nayak (Biology), David A. Hunt
(Chemistry) - $60,000
TCNJ SOSA Grant Award: release time for academic year (2006-2007; 2007-2008; 2008-2009).
Bristol-Myers Squibb Undergraduate Research Award in Organic Chemistry -
(Summers, 2006; 2007) - $10,000
National Starch Summer Research Grant (2007) - $5,000; 2 students (2008) -
$10,000.
TCNJ
SURP Grant for 3 research students (2006) - $6,000; 1 student (2007) - $2,500
TCNJ
MUSE Grant for 2 research students (2008; 2012, 2013) - $10,000
NASA
(New Jersey Space Grant Consortium) Summer
Research Award (2011) - $5,000
Students
Ms. Joanne Bertanozzi (2006 BMS Grant Recipient - Summer, 2006):
Preparation of Novel Ring Systems Utilizing Parham Cycliacylation/ Cyclialkylation Chemistry (Summer, 2006)
Ms. Jessica Bocanegra
Michael Reactions with Knovenagel Adducts (Fall, 2013; Spring, 2014)
Ms. Catherine Campos:
Studies Directed Towards Synthesis of Benzo-fused Oxepinones and Thiapanones (Spring 2006 - Spring, 2007)
Ms. Emily Cherney:
Tandem Cyclization of Amino Acid Amides (Fall, 2005)
Studies Toward a Novel Synthesis of Dihydroquinolines (Spring, 2006)
Solvent Effects on the Preparation of 1,2,3,4-Tetrahydrobenzodiazapin-5-ones (Fall, 2006 - Spring, 2007)
Mr. Keith Chomsky (2007 BMS Grant Recipient - Summer, 2007):
Lithium-Halogen Exchange Behavior in Bromo-Substituted 1,4-Diaryl b-Lactams (Spring, 2007; Summer, 2007; Fall, 2007 - Spring, 2008)
Mr. Adam Clarke:
A Novel Synthesis of 1-Phenyl-2-Nitroindenes (Spring, 2006 - Fall, 2006)
Mr. Tim Craven:
Toward a Novel Synthesis of the Benzopyrazole Ring System via Condensation of Thermolabile Aryllithium Reagents and Diazodicarboxylate Esters (Summer, 2006)
Ms. Kate Davis:
A New Route to Styryl Vinyl Ethers (Summer, 2007; Summer 2008; Fall, 2008 - Spring, 2009)
Ms. Sara Davis:
A Total Synthesis of (+)-Isopestacin (Fall, 2007 - Fall, 2008)
Mr. Ryan DeAngelis
New Intramolecular Cyclization Strategies Built
Around Novel Parham-type Substrates
Mr. John Farrokh (New Jersey Space Grant Consortium Fellow, Summer, 2011):
Studies Directed Towards Synthesis of Benzo-fused Oxepinones and Thiapanones (Summer, 2011; Fall, 2011- Spring, 2012)
Toward a Novel Synthesis of the Benzopyrazole
Ring System via Condensation of Thermolabile Aryllithium Reagents and Diazo-
dicarboxylate Esters (Fall, 2012; Spring, 2013)
Ms. Brittany Frazier:
A Novel Synthesis of 2-Nitro-3-Arylindanones (Fall, 2009 - Spring, 2010)
Mr. Alex Fuchs:
A Novel Synthesis of 2-Nitro-3-Arylindanones (Fall, 2009 - Spring, 2010)
Ms. Maryll Geherty:
Lithium-Halogen Exchange Behavior in Bromo-Substituted 1,4-Diaryl b-Lactams (Summer, 2006)
Ms. Amber Gietter:
A Novel Synthesis of 2-Nitro-3-Arylindanones (Fall, 2009 - Spring, 2010)
Mr. Tyler Higgins
Addition of 1,2-Cyclohexanedione to a Variety of Michael Acceptors (Summer, 2012; Fall, 2012; Spring, 2013)
Preparation of Resveratrol Derivatives as Potential CNS Agents (Summer, 2013; Fall, 2013; Spring, 2014)
Ms. Jenna Klubnick:
A Total Synthesis of (+)-Clavulazine (Fall, 2007- Spring, 2008)
Mr. Joe Macor:
Tandem Cyclization of Amino Acid Amides (Summer, 2008)
Ms. Taylor Maney
Heck Reactions with Highly Functionalized Bromoarenes (Fall, 2013)
Preparation of Resveratrol Derivatives as Potential CNS Agents (Summer, 2013; Fall, 2013; Spring, 2014)
Mr. Jim Melnyk:
Lithium-Halogen Exchange Behavior in Bromo-Substituted 1,4-Diaryl b-Lactams (Spring, 2007 - Spring, 2008)
Ms. Christina Papanagapoulous:
Tandem Cyclization of Amino Acid Amides (Fall, 2005, Fall, 2006)
Cyclization Reactions of Amino Acid Amides (Spring, 2006)
Reaction of Functionalized Aryllithium Reagents with Trialkyl- and Triarylboranes (Summer, 2006)
Mr. Mike Rosana (National Starch Grant Recipient - Summer, 2007):
Expedient Preparation of 2-(5,6-Dihydro-4H-1,3-oxazin-2-yl)anilines (Summer, 2007 - Fall, 2007; Spring, 2008)
Ms. Marissa Rubenstein:
Cyclization Strategies Built Around
b-Nitro-substituted
Systems as Michael Acceptors
Mr. Chad Simpkins:
Michael Additions of b-Nitrostyrenes to1,2-Cyclohexanedione (Fall, 2010 - Spring, 2012; Summer, 2011)
Ms. Amy Solinski
Condensation/Aromatization Reactions with 1,2-Cyclohexanedione (Fall, 2013; Spring, 2014)
Ms. Sarah Thornton:
Reaction of Functionalized Aryllithium Reagents with Isatoic Anhydrides (Fall,
2010 - Spring, 2012)
Ms. Erica Tabakin (Merck/AAAS Research Undergraduate Research Fellow - Summer, 2008):
Development of Small Molecule Proteasome Inhibitors (Summer, 2007; Summer 2008)
Preparation of Some 4,6-difluoro-3-arylisobenzofuran-1(3H)-ones (Fall. 2008 - Spring, 2009)
Ms. Kelsey VanGelder (Merck/AAAS Research Undergraduate Research Fellow - Summer, 2009):
A Convergent Synthesis of (+)-Isopestacin (Summer, 2009; Fall, 2009; Spring, 2010-Spring, 2011)
Ms. Lyndsay Wood (Merck/AAAS Research Undergraduate Research Fellow - Summer, 2009):
Development of Small Molecule Proteasome Inhibitors (Summer, 2009)
Where Are They Now? Former Undergraduate Research Students
Catherine Campos (2007) - Notre Dame (Ph.D. - Ashfeld)
Emily Cherney (2007) - Scripps (Ph.D - Baran); Medicinal Chemistry- BMS (Princeton)
Maryl Geherty (2007) - Pittsburgh (Ph.D. - Nelson); Princeton (postdoc - Sorensen)
Joanne Bertonazzi (2008) - Pittsburgh (Ph.D. - Nelson)
Keith Chomsky (2008) - Unknown
Adam Clarke (2008) - Bristol-Myers Squibb (medicinal chem)
Jenna Klubnick (2008) - Illinois [M.S. (2011) - organic; Burke]; Harvard (Center for Systems Biology, Weissleider)
Jim Melnyk (2008) - Delaware (organic/biochem; Grimes)
Mike Rosana (2008) - Florida State (organic; Dudley)
Tim Craven (2009) - NYU (Kirshenbaum)
Kate Davis (2009) - Dow Chemical
Sara Davis (2009) - Hohenheim (agriculture)
Erica Tabakin (2009) - Robert Wood Johnson Medical School (M.D.); Hospital of the University of Pennsylvania - Emergency Medicine
Brittany Frazier (2010) - Northeastern (M.S.N program)
Alex Fuchs (2010) - Connecticut (dental school)
Amber Gietter (2010) - Delaware (organic; Watson, Don)
Joe Macor (2010) - Illinois (materials; organic)
Lyndsay Wood (2010) - Pennsylvania (organic; Winkler)
Kelsey VanGelder (2011) - Pennsylvania (organic; Kozlowski)
Chad Simpkins (2012) - Robert Wood Johnson Medical School
Sarah Thornton (2012) - Jefferson Medical College
John Farrokh (2013) - UNC - Chapel Hill (organic, Johnson)
Ryan DeAngelis (2014) - Cooper Medical School (NJ)
Tyler Higgins (2014) - Pennsylvania (organic; TBD)
Marissa Rubenstein (2014) - Rutgers Dental School